-
Oxygen reduction reaction (ORR) is a pivotal process in clean renewable energy conversion systems such as fuel cells and metal-air batteries[1-2]. Until now, platinum (Pt) and platinum-based alloys have been proven to be the most efficient catalysts for catalyzing intrinsically sluggish ORR, however, the high cost of Pt significantly hamper their broad applications[3]. Therefore, developing high performance and stable non-precious-metal catalysts (NPMCs) for ORR is of great importance to promote the commercialization of fuel cells.
To date, many efforts have been devoted to developing NPMCs to substitute Pt-based catalysts, including metal-nitrogen-carbon (M-N-C)[4-5], transition metal oxide[6], and heteroatom-doped carbon materials[7]. Among them, the M-N-C materials have been highlighted as promising candidates owing to their low cost, environmental friendliness and high catalytic activity, as well as good durability[8-9]. In genera l, M-N-C catalysts are synthesized by direct pyrolysis of nitrogen-containing carbon (NC) precursors with the aid of transition metal (i.e. Fe, Co, etc.) at a high temperature (600~1 000 ℃)[10]. Although the direct-pyrolysis method is simple and effective, accurate regulation of the distribution of the active sites is difficult and would result in many active sites embedding inside the M-N-C catalysts, which are not easily accessible to the reactants (O2), thus leading to a low ORR efficiency[11]. Moreover, the poor electrical conductivity of the as-obtained M-N-C is unfavorable for the electrocatalytic process. To make the active sites of the catalysts exposed and improve the conductivity of the catalyst, one-dimensional carbon nanotubes (CNTs) have been widely used as the support of the catalyst[12-13]. The CNT supports not only possess hierarchical pore structure to promote mass transport, but also provide long electron transport highways to increase the electron transfer efficiency. However, the common oxidative acid treatment of CNTs will destroy its sidewalls, which is undesirable for electron conduction. Furthermore, the uneven dispersion of Fe and Co salt precursor on the surface of CNTs via physical adsorption would lead to non-uniform distribution of Fe-Nx active sites after heat treatment. Thus, it is highly desirable to develop a simple and versatile method to load NC precursor on the surface of CNTs for preparing M-N-C catalyst.
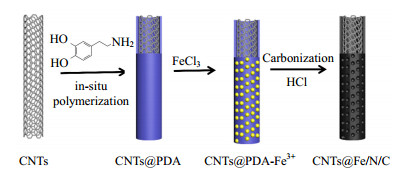
Herein, we report a facile polydopamine (PDA) modification approach to prepare porous CNTs@Fe/N/C elecrtocatalysts. As shown in Fig. 1, CNTs without any pretreatment was firstly immersed into the dopamine solution followed by the addition of Tris-buffer (pH≈8.5) solution, and the dopamine will spontaneously polymerize and form a hydrophilic PDA layer firmly adhering to CNTs. Then, Fe (Ⅲ) ions (FeCl3) were uniformly decorated on the surface of CNTs@PDA via the coordination between Fe3+ and -OH in PDA. After carbonization, the resulting CNTs@Fe/N/C catalyst exhibited enhanced ORR catalytic activity and excellent stability in alkaline medium.
-
100 mg of CNTs without having any pretreatment was dispersed in the solution mixtures of deionized water (40 mL) and ethanol (30 mL). Dopamine (200 mg) in Tris-HCl (30 mL, 50 mM, pH≈8.5) solution was then added. The reaction was allowed to proceed under magnetic stirring for 24 h at room temperature. The obtained CNTs@polydopamine (CNTs@PDA) nanocomposite was collected by centrifugation and washed with deionized water and then dried at 60℃ for 12 h. Then, the CNTs@PDA was stirred with FeCl3 in deionized water to coordinate Fe (Ⅲ) ions with -OH in PDA structure. After pyrolysis of CNTs@PDA-Fe under N2 atmosphere for 2 h at 800 ℃, the CNTs@Fe/N/C catalysts were obtained by HCl washing. The resulting materials were designated as "CNTs@XFe/N/C", where X represents the weight ratio of FeCl3 to CNTs@PDA (X =0, 20%, 40% and 60%).
-
Transmission electron microscopy (TEM) was carried out on a Zeiss LIBRA 200 FETEM instrument operating at 200 kV. Raman spectrum was recorded by a LabRamHR evolution Raman spectrometer equipped with an Nb-Yag laser excitation source operated at 532 nm. X-ray Photoelectron Spectroscopy (XPS) was conducted on a PE PHI-5400 spectrometer equipped with a monochromatic Al X-ray source (Al KR, 1.486 6 keV).
-
All electrochemical experiments were performed in a standard three-electrode cell at room temperature. The cell consisted of a glassy carbon working electrode (GC electrode, d=5 mm), an Ag/AgCl reference electrode, and a carbon rod counter electrode. All potentials in this study are given relative to a reversible hydrogen electrode (RHE). The working electrodes were prepared by applying catalyst ink onto glassy carbon (GC) disk electrodes. The loading of the as-prepared catalyst is 0.5 mg·cm-2.
All of the electrodes were pretreated by cycling between 0 and 1.2 V (vs. RHE) at a sweep rate of 50 mV·s-1 for 50 cycles in order to remove any surface contamination prior to ORR activity testing.
The activities of catalysts were performed by recording linear sweep voltammetry (LSV) curves in the oxygen-saturated 0.1 M KOH solution. The LSV curves for ORR were recorded at potential scan rate of 10 mV·s-1. The rotation speed was controlled at 1 600 rpm.
Rotating disk electrode (RDE) measurements were conducted at rotation speeds from 100 to 2 500 rpm using a VersaSTAT3 (V3). RDE measurements were conducted in O2-saturated 0.1 M KOH solution at 10 mV·s-1 scan rate. The electron transfer number (n) was analysed on the basis of Koutecky-Levich equations shown in equations:
$$\frac{1}{J} = \frac{1}{{{J_D}}} + \frac{1}{{{J_K}}} = \frac{1}{{B{\omega ^{1/2}}}} + \frac{1}{{{J_K}}}$$ (1) $$B = 0.62nF{C_0}{({D_0})^{2/3}}{\upsilon ^{ - 1/6}}$$ (2) in which J is the measured current density, JK and JD are the kinetic- and diffusion-limiting current densities, $\omega $ is the angular velocity of the disk ($\omega = 2\pi V$, N is the linear rotation speed), n is the overall number of electrons transferred in ORR, F is the Faraday constant (F = 96 485 C·mol-1), C0 is the bulk concentration of O2, ʋ is the kinematic viscosity of the electrolyte, and k is the electron transfer rate constant. According to Equations (1) and (2), the number of electrons transferred (n) and JK can be obtained from the slope and intercept of the Koutecky-Levich plots, respectively, by using the values of C0 = 1.2×10-3 mol·L-1, D0 = 1.9×10-5 cm s-1 and $\upsilon $ = 0.1 m2·s-1 in 0.1 M KOH.
The CV accelerating stress tests (AST) were performed at potentials between 0 and 1.2 V vs. RHE at a scan rate of 50 mV·s-1 in nitrogen-purged 0.1 M KOH at room temperature. RDE cycling stability tests of the catalysts were performed in N2-saturated 0.1 M KOH in the potential range of 0 to 1.2 V vs. RHE.
-
The morphology of the samples was first investigated by transmission electron microscopy (TEM). The typical TEM image in Fig. 2a evidences that the product has cable-like one-dimensional (1D) structures. Under a higher magnification (Fig. 2b), the core region of the 1D structure can be identified to be CNTs, and the surface was uniformly covered by a layer of NC with a thickness of~6 nm. Fig. 2c shows that the morphology of the product does not change much after Fe introduction, except that the thickness of the PDA layer is slightly decreased to~4 nm. This could be ascribed to the decomposition and gasification of PDA with the aid of FeCl3 at 800 ℃. Moreover, high-resolution TEM image in the insert of Fig. 2d clearly reveals the lattice fringes in the core region have an interplanar distance of ca. 0.34 nm, corresponding to the (002) plane of graphitic carbon. On the other hand, the outer sheath is more disordered, suggesting the amorphous nature of the carbon coating layer[14]. These CNTs could serve as a three- dimensional conductive network in catalysts which facilitates the electron migration.
The defected structures of CNTs@Fe/N/C were evaluated by Raman spectra. As shown in Fig. 3a, there are two peaks at around 1 340 cm-1 and 1 590 cm-1 for all as-prepared samples, assigned to D band and G band of graphite carbon, respectively. The D band suggests the existence of substantial defects or disordered sites in the composite, whereas the G band refers to the graphitic structure. The calculated ID/IG ratio for CNTs@Fe/N/C catalyst is about 1.09~1.15, higher than that for CNTs@N/C (1.07) samples, implying that the number of defects in CNTs@Fe/N/C increased after the Fe introduction during the carbonization process.
The porous nature of these catalysts was further characterized by N2 adsorption-desorption experiments. The adsorption isotherm obtained for CNTs@Fe/N/C catalyst exhibits a behavior of type-IV with H4 hysteresis loop (Fig. 3b) with all the important values summarized in Table 1. The Brunauer-Emmett-Teller (BET) specific surface area of pristine CNTs was measured to be only 84.3 m2·g-1. When the pyrolysis process was carried without Fe, CNTs@N/C shows a very low SBET of only 137.9 m2·g-1, suggesting a poorly developed porosity. With the increasing Fe content, the SBET dramatically increases from 213.6 m2·g-1 for CNTs@20%Fe/N/C to 301.4 m2·g-1 for CNTs@40%Fe/N/C, and it drops to 256.5 m2·g-1 for CNTs@60%Fe/N/C (Table 1). The pore size distribution curves of CNTs@Fe/N/C (Fig. 3c, 3d) exhibit sharp peaks centered at about 2.0 and 15 nm, confirming their mesoporous structures. Remarkably, the high BET surface area and the enriched porous structure are favorable for mass transfer of reactants and products, and able to offer more exposed active sites. All these profitable features could contribute to an enhanced ORR activity.
Table 1. Porous structure and elements analysis pristine CNTs and CNT@ Fe/N/C
Samples Surface area /m2·g-1 Pore volume /cm3·g-1 Average pore size /nm N Content /at.% Fe Content /at.% Pyridinic-N /% Pyrrolic-N /% Graphitic-N /% Pristine CNTs 84.3 — — — — — — — CNTs@ N/C 137.9 0.245 0.71 2.25 --- 29.4 31.4 39.2 CNTs@20% Fe/N/C 213.6 0.464 1.65 1.80 0.37 43.3 27.7 29.0 CNTs@40% Fe/N/C 301.4 0.422 1.81 1.47 0.41 45.9 26.3 27.8 CNTs@60% Fe/N/C 256.5 0.238 1.98 1.12 0.42 40.2 28.7 31.1 The chemical states of the elements of these samples were determined by X-ray Photoelectron Spectroscopy (XPS). The relative contents of N element and different N species of CNTs@N/C and CNTs@Fe/N/C catalysts are summarized in Table 1. It is apparent that the N content of CNTs@N/C without Fe remains at a high level around 2.25 at%, however, when the Fe content is increased from 20% to 60%, the N content exhibits a gradually decreasing trend. It can be observed that the increase of Fe content will increase the BET specific surface area of the materials and lead to the decrease of N doping content, which is consistent with our previously reported result[15]. The N 1s XPS spectrum (Fig. 5c) can be deconvoluted into three main peaks centered at 398.6, 400.5, and 401.3 eV, which can be assigned to pyridinic-N, pyrrolic-N and graphitic-N, respectively. These N species play a very important role as an active center for ORR[16-18].
The ORR catalytic activities of the as-prepared catalysts were first investigated using cyclic voltammetry (CV) in 0.1 M KOH electrolyte. As shown in Fig. 5a, the CV curve of CNTs@40%Fe/N/C obtained in O2-saturated KOH electrolyte displayed a well-defined cathodic peak at approximately 0.81 V, which is absent in the CV obtained in the N2-saturated electrolyte, indicating its excellent electrocatalytic activity for ORR. To investigate its comprehensive ORR catalytic activity, linear sweep voltammetry (LSV) curves of CNTs@Fe/N/C and commercial Pt/C catalyst were obtained using a rotating disk electrode (RDE). The ORR activity of CNTs@Fe/N/C increases quickly with the increasing Fe content and reaches the maximum for CNTs@40%Fe/N/C with regard to both diffusion limiting current density at 0.4 V (5.12 mA cm-2) and half-wave potential (0.881 V), which is comparable to that of commercial Pt/C. Further increasing the Fe content to 60% leads to a slight decrease in the onset/half-wave potential of the CNTs@60%Fe/N/C catalyst, which can be ascribed to the decreased specific surface area (see Table 1). The superior ORR activity of CNTs@40%Fe/N/C can thus be attributed to the optimal exposure of the ORR active sites as a result of the porous micro/mesoporous structure.
To gain further insight into the electron transfer process in ORR catalysis of the CNTs@40%Fe/N/C catalyst, a serial of RDE measurements on CNTs@ 40%Fe/N/C in 0.1 M KOH at different rotating speeds was carried out (Fig. 5c). Fig. 6d presents the Koutecky-Levich (K-L) plots at 0.40 V, 0.50 V and 0.60 V calculated from the LSV curves at the various rotating speeds (100~2 500 rpm). The K-L lines for 0.40 V, 0.50 V and 0.60 V are almost superimposed, indicating that ORR occurs on these potentials in a similar way. The average number of transferred electrons during the ORR was 3.50, indicating the near four electron selectivity of the catalyst in alkaline media.
The stabilities and possible crossover effects of catalytic materials are also very important for practical applications. The LSV curves of the CNTs@40%Fe/ N/C was evaluated at 10 mV·s-1 in an O2-saturated 0.1 M KOH solution as well as in an O2-saturated 0.1 M KOH solution with the addition of 0.5 M CH3OH. As shown in Fig. 5e, no noticeable changes were observed in the ORR polarization curves on CNTs@40%Fe/N/C catalyst after adding 0.5 M CH3OH. This implies that this catalyst has a high selectivity for ORR with a remarkable ability to avoid the crossover effect and holds high promise for use in direct methanol fuel cells. Meanwhile, the stability of the CNTs@40%Fe/N/C catalyst was determined before and after proper potential sweeps between 0 and 1.2 V with a scan rate of 50 mV·s-1 in N2-saturated 0.1 M KOH. The ORR half-wave potential still remained the same as the original value after 5 000 cycles and only the diffusion limiting current decreases ca. 0.26 mA·cm-2, evidently highlighting the outstanding electrocatalytic stability of the sample for ORR in an alkaline electrolyte.
-
In summary, we reported a simple method to synthesize core-shell nanowire CNTs@Fe/N/C as an efficient electrocatalyst for ORR. The CNTs@Fe/N/C catalysts are composed of CNT cores and porous Fe/N-doped carbon shells derived by pyrolysis of PDA at 800 ℃ with the aid of FeCl3. The CNT core plays a role of a three-dimensional conductive network for fast electron transfer. At the same time, porous Fe/N-doped carbon layer offers numerous catalytic active sites and fast mass transfer for ORR. Benefited from the well-defined structure and rich actives sites, the CNTs@40%Fe/N/C catalyst presented an ORR activity comparable to the commercial Pt/C in alkaline media and also showed superior durability and resistance to the methanol crossover. This work may shed light on the future development of practical non-noble metal- based catalysts for fuel cells.
Porous Fe/N-doped Carbon Layers Wrapped CNTs Electrocatalysts for Superior Oxygen Reduction Reaction
-
摘要: 开发高性能、低成本的氧还原催化剂是降低燃料电池成本的关键之一。过渡金属-氮-碳材料具有催化活性高、成本低、环境友好等优点,被认为具有广阔的应用前景。该文提出了一种简单的聚多巴胺改性碳纳米管的方法,在碳纳米管(CNTs)表面包覆聚多巴胺(PDA),通过高温裂解CNTs@PDA和FeCl3复合物制备多孔CNTs@Fe/N/C电催化剂。用TEM、BET、Raman和XPS对制备的催化剂的形貌和组成进行了表征。电化学结果表明,CNTs@40% Fe/N/C催化剂的半波电位高达0.881 V,接近于商业化Pt/C催化剂。此外,CNTs@40% Fe/N/C催化剂亦具备优异的抗甲醇干扰性及稳定性,是一种有良好实际应用前景的燃料电池非贵金属氧还原电催化剂。
-
关键词:
- CNTs /
- electrocatalyst /
- Fe/N/C /
- oxygen reduction reaction /
- polydopamine
Abstract: Developing high performance, low-cost oxygen reduction electrocatalysts is the key to reduce the cost of fuel cells. The transition metal-nitrogen-carbon materials have been highlighted as promising candidates owing to their high catalytic activity, low cost and environmental friendliness. Herein, we report a facile polydopamine modification approach to prepare porous CNTs@Fe/N/C elecrtocatalysts via pyrolysis of the CNTs@PDA and FeCl3 composites. The morphology and composition of these as-prepared catalysts were characterized by TEM, BET, Raman and XPS. The electrochemical results show that the half-wave potential of the CNTs@40% Fe/N/C catalyst is as high as 0.881 V, which is close to that of commercial Pt/C catalyst. Moreover, the CNTs@40% Fe/N/C also showed superior durability and resistance to the methanol crossover.-
Key words:
- CNTs /
- electrocatalyst /
- Fe/N/C /
- oxygen reduction reaction /
- polydopamine
-
Table 1. Porous structure and elements analysis pristine CNTs and CNT@ Fe/N/C
Samples Surface area /m2·g-1 Pore volume /cm3·g-1 Average pore size /nm N Content /at.% Fe Content /at.% Pyridinic-N /% Pyrrolic-N /% Graphitic-N /% Pristine CNTs 84.3 — — — — — — — CNTs@ N/C 137.9 0.245 0.71 2.25 --- 29.4 31.4 39.2 CNTs@20% Fe/N/C 213.6 0.464 1.65 1.80 0.37 43.3 27.7 29.0 CNTs@40% Fe/N/C 301.4 0.422 1.81 1.47 0.41 45.9 26.3 27.8 CNTs@60% Fe/N/C 256.5 0.238 1.98 1.12 0.42 40.2 28.7 31.1 -
[1] WU G, MORE K L, JOHNSTON C M, ZELENAY P. High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt[J]. Science, 2011, 332(6208):443-447. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0224113762/ [2] NIE Y, LI L, WEI Z D. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction[J]. Chemical Society Reviews, 2015, 44:2168-2201. doi: 10.1039/C4CS00484A [3] WANG Q, CHEN S, SHI F, et al. Structural evolution of solid pt nanoparticles to a hollow ptfe alloy with a pt-skin surface via space-confined pyrolysis and the nanoscale kirkendall effect[J]. Advanced Materials, 2016, 28(48):10673-10678. doi: 10.1002/adma.201603509 [4] WAN X, LIU X, LI Y, et al. Fe-N-C electrocatalyst with dense active sites and efficient mass transport for high-performance proton exchange membrane fuel cells[J]. Nature Catalysis, 2019, 2(3):259-268. doi: 10.1038/s41929-019-0237-3 [5] RATSO S, RANJBAR SAHRAIE N, SOUGRATI M T, et al. Synthesis of highly-active Fe-N-C catalysts for PEMFC with carbide-derived carbons[J]. Journal of Materials Chemistry A, 2018, 6(30):14663-14674. doi: 10.1039/C8TA02325E [6] LI A, WANG C W, ZHANG H N, et al. Graphene supported atomic Co/nanocrystalline Co3O4 for oxygen evolution reaction[J]. Electrochimica Acta, 2018, 276:153-161. doi: 10.1016/j.electacta.2018.04.177 [7] JIANG W J, HU J S, ZHANG X, et al. In situ nitrogen-doped nanoporous carbon nanocables as an efficient metal-free catalyst for oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2014, 2(26):10154-10160. doi: 10.1039/c4ta01780c [8] TANG Z, ZHAO Y, LAI Q, et al. Stepwise fabrication of co-embedded porous multichannel carbon nanofibers for high-efficiency oxygen reduction[J]. Nano-Micro Letters, 2019, 11(1):1. http://d.old.wanfangdata.com.cn/Periodical/wnkb-e201902015 [9] KASATKIN P E, JÄGER R, HÄRK E, et al. Fe-N/C catalysts for oxygen reduction based on silicon carbide derived carbon[J]. Electrochemistry Communications, 2017, 80:33-38. doi: 10.1016/j.elecom.2017.05.001 [10] WU R, WANG J, CHEN K, et al. Space-confined pyrolysis for the fabrication of Fe/N/C nanoparticles as a high performance oxygen reduction reaction electrocatalyst[J]. Electrochimica Acta, 2017, 244:47-53. doi: 10.1016/j.electacta.2017.04.169 [11] WU R, SONG Y, HUANG X, et al. High-density active sites porous Fe/N/C electrocatalyst boosting the performance of proton exchange membrane fuel cells[J]. Journal of Power Sources, 2018, 401:287-295. doi: 10.1016/j.jpowsour.2018.08.096 [12] NIE Y, XIE X, CHEN S, et al. Towards effective utilization of nitrogen-containing active sites:nitrogen-doped carbon layers wrapped cnts electrocatalysts for superior oxygen reduction[J]. Electrochimica Acta, 2016, 187:153-160. doi: 10.1016/j.electacta.2015.11.011 [13] ZHANG Y, JIANG W J, GUO L, et al. Confining iron carbide nanocrystals inside CNx@CNT toward an efficient electrocatalyst for oxygen reduction reaction[J]. ACS Appl Mater Interfaces, 2015, 7(21):11508-11515. doi: 10.1021/acsami.5b02467 [14] HU F, YANG H, WANG C, et al. Co-N-Doped mesoporous carbon hollow spheres as highly efficient electrocatalysts for oxygen reduction reaction[J]. Small, 2017, 13(3):1-8. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=492ae7da40fc3fc0b4ce053beadbf969 [15] WU R, CHEN S, ZHANG Y, et al. Controlled synthesis of hollow micro/meso-pore nitrogen-doped carbon with tunable wall thickness and specific surface area as efficient electrocatalysts for oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2016, 4(7):2433-2437. doi: 10.1039/C5TA09859A [16] ZAHOOR A, CHRISTY M, HWANG Y J, et al. Improved electrocatalytic activity of carbon materials by nitrogen doping[J]. Applied Catalysis B:Environmental, 2014, 147:633-641. doi: 10.1016/j.apcatb.2013.09.043 [17] CAI S, MENG Z, TANG H, et al. 3D Co-N-doped hollow carbon spheres as excellent bifunctional electrocatalysts for oxygen reduction reaction and oxygen evolution reaction[J]. Applied Catalysis B:Environmental, 2017, 217:477-484. doi: 10.1016/j.apcatb.2017.06.008 [18] MENG J, NIU C, XU L, et al. General oriented formation of carbon nanotubes from metal-organic frameworks[J]. Journal of the American Chemical Society, 2017, 139(24):8212-8221. doi: 10.1021/jacs.7b01942 -

 ISSN
ISSN 




 下载:
下载: