-
印制电路板(printed circuit board, PCB)广泛存在于各种电子产品中,用于电子元器件的安装和互连。随着电子信息技术的飞速发展及生产方式的绿色转型,PCB的制造越来越受到关注[1-3]。目前,传统的蚀刻工艺在PCB制作中仍占据主导地位,但这种方法存在工艺复杂、铜箔浪费和环境污染等缺点[4-5]。因此,研究工艺简单、不产生任何材料浪费的加成工艺是PCB制作未来的发展方向。
选择性化学镀铜法能在树脂基材表面直接沉积铜导电线路,属于加成工艺制作PCB的一种常用方法[6]。然而树脂自身的惰性使其不能催化铜沉积,同时树脂低的表面能使其难于固定催化剂[7]。针对树脂的惰性和低表面能,大量文献研究了使用表面粗糙化、共价接枝、胶粘材料、激光处理等方法对树脂基材表面改性并固定催化剂[8-11]。尤其,树脂基材表面共价接枝改性层,借助改性层吸附催化剂是目前的研究热点。文献[12]在树脂基材表面聚合一层聚多巴胺,聚多巴胺自身具有吸附和还原Ag+的能力,通过选择性化学镀铜法制作了性能优异的导电线路。文献[13]在树脂基材表面共价接枝含特殊官能团的聚合物,借助聚合物表面的特殊官能团吸附催化剂前驱体Pd2+,通过可控的选择性化学镀铜法沉积性能优异的导电线路和器件。共价接枝上述改性层虽然能够催化铜导电线路在树脂基材表面沉积,但通常改性层与树脂基材的物理和化学性质不同,电子元器件产生的热量会导致改性层与树脂基材之间产生应力,影响铜线路与树脂基材之间的结合力。
双酚A二缩水甘油醚(bisphenol A diglycidyl ether, BADGE)是环氧树脂(epoxy resin, EP)的一种预聚体,本研究选用BADGE、固化剂593、硫脲、乙酸铜和丙二醇甲醚为原料,设计了一种基于EP兼容的Cu2+溶液。
-
选用量子化学计算模拟硫脲分子与Cu2+的吸附位点,分析不同比例硫脲分子与Cu2+形成络合物的稳定性,为兼容性Cu2+溶液的配制提供理论指导。兼容性Cu2+溶液中硫脲分子与Cu2+之间的络合反应,选用密度泛函B3LYP方法进行模拟[14-16]。硫脲分子与Cu2+形成络合物的结构优化,选用6-311G+ (d, p)基组对H、C、N和S元素进行计算,选用LANL2DZ基组对Cu元素进行计算[17]。
络合物中硫脲分子与Cu2+之间的键能为[15]:
式中 ,
$ {{E}}_{{\text{Cu}}^{{2+}}} $ 是Cu2+的能量;$ {{E}}_{\text{thiophene}} $ 是硫脲分子的能量;$ {{E}}_{\text{complex}} $ 是硫脲分子与Cu2+形成络合物的总能量。 -
丙二醇甲醚(C4H10O2, 99.5%)购买于上海麦克林生化科技有限公司;BADGE(C21H24O4)购买于西格玛奥德里奇(上海)贸易有限公司;硫脲(CH4N2S, 99.0%)、乙酸铜(C4H6CuO4·H2O,99.0%)购买于成都市科龙化工试剂厂;固化剂593(C11H27N3O)购买于郑州祥之达化工有限公司。兼容性Cu2+溶液制备过程如下:首先,将硫脲(0.004 mol)和丙二醇甲醚(10 mL)依次加入玻璃样品瓶(15 mL),磁力搅拌(室温,200 rpm)10 min得到无色透明溶液;然后,往玻璃样品瓶中加入乙酸铜(0.002 mol),继续磁力搅拌直到乙酸铜完全溶解得到蓝色透明溶液;接着,往玻璃样品瓶中加入BADGE(0.02 mol),保持磁力搅拌30 min得到蓝色透明溶液;最后,往玻璃样品瓶中加入固化剂593(0.004 mol),继续磁力搅拌30 min得到蓝色透明的兼容性Cu2+溶液。
-
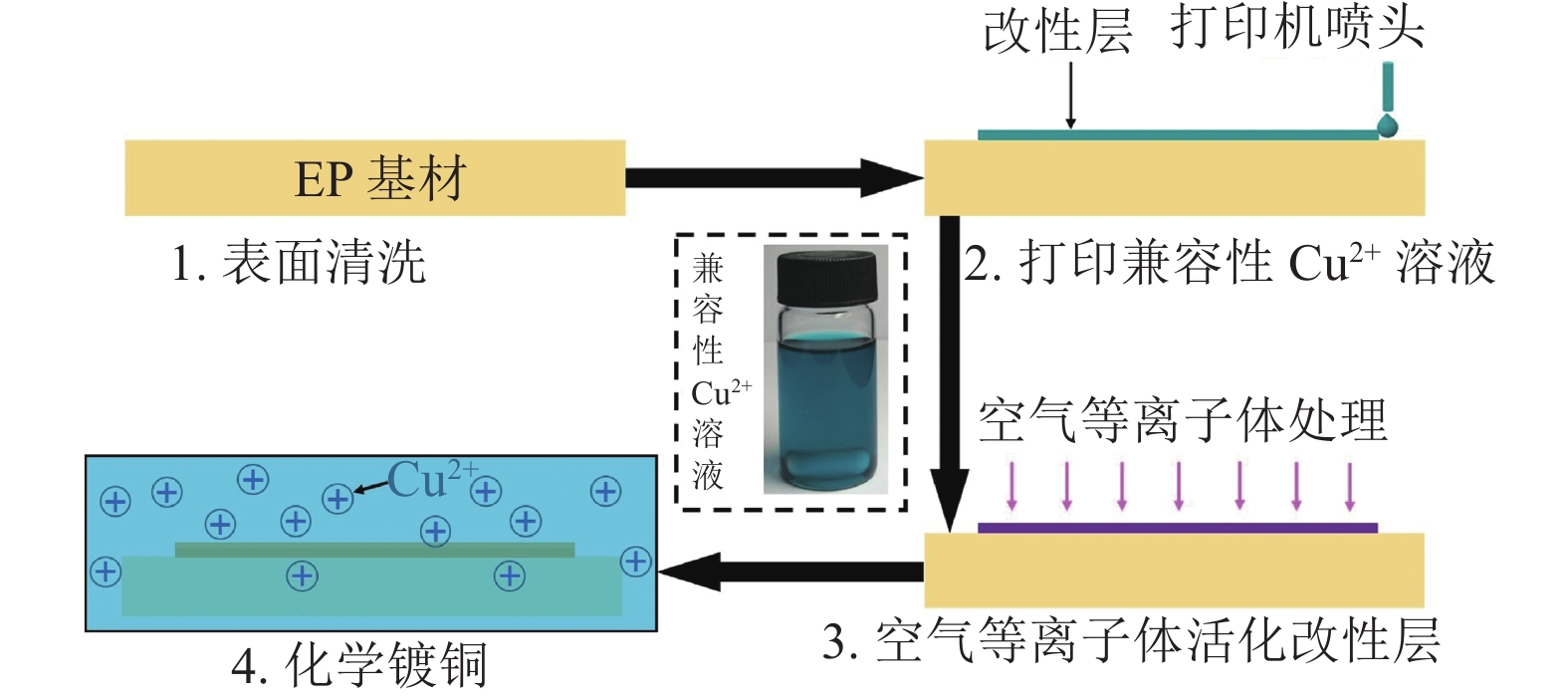
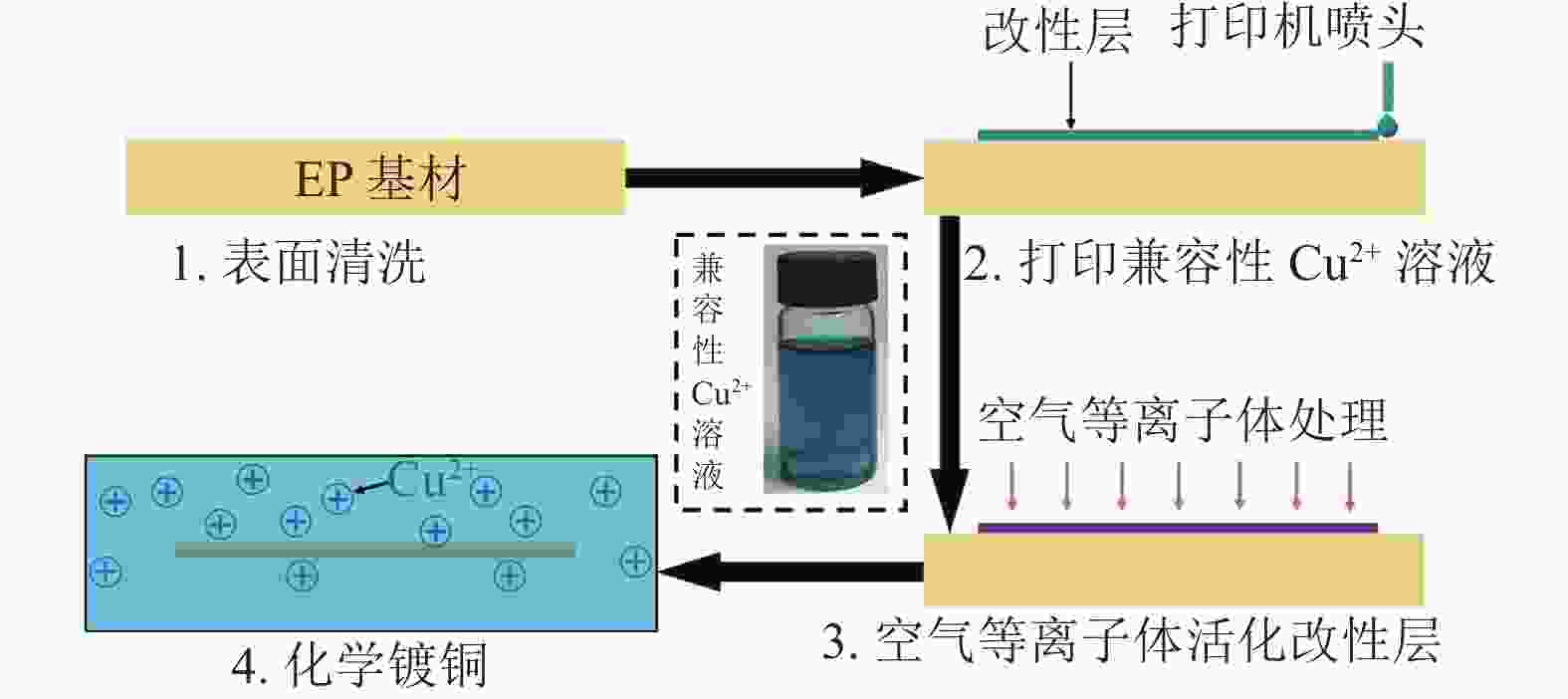
图1为兼容性Cu2+溶液改性EP基材催化铜导电线路沉积的工艺流程图。分为4个步骤:1)表面清洗:室温下把EP基材浸入盛有乙醇的烧杯中,用超声波清洗仪(CPX2800H-C)处理5 min,然后用去离子水冲洗3遍,接着放入烘箱中(80 °C)干燥30 min后得到表面干净的EP基材。2)表面改性:借助喷墨打印机把插图中的兼容性Cu2+溶液印刷在干净的EP基材表面形成预设电路图形,待兼容性Cu2+溶液形成的预设电路图形固化后转变为改性层。3)活化:把改性后的EP基材放入空气等离子体(220 V, 40 kHz, 150 W)处理15 min,使改性层中的Cu2+被还原为具有催化活性的Cu纳米颗粒。4)化学镀铜:选用先前工作使用的化学镀铜液配方和工艺[18],把活化后的EP基材浸入到化学镀铜液中进行选择性化学沉铜,得到所需的铜导电线路。
-
红外光谱仪(thermo fisher scientific)和拉曼光谱仪(Horiba iHR550)测试兼容性Cu2+溶液中特殊官能团的变化;改性层中铜元素化合价借助X射线光电子能谱仪(X-ray photoelectron spectroscopy, XPS, THERMO ESCALAB 250XI )进行表征;选用百格测试法评估铜导电线路与EP基材之间的结合力;原子力显微镜(atomic force microscopy, AFM, dimension ICON)测试EP基材表面粗糙度;X射线衍射仪(PHILIPS X’PERT MPD)表征化学沉积层的成分和结晶度;扫描电子显微镜(scanning electron microscopy, SEM, HIEACHI S3400)表征改性层和铜线路的表面形貌;台阶仪(Dektak XT)测量铜导电线路的厚度;多功能电表(Keithley 2400)测试铜导电线路的电阻。
-
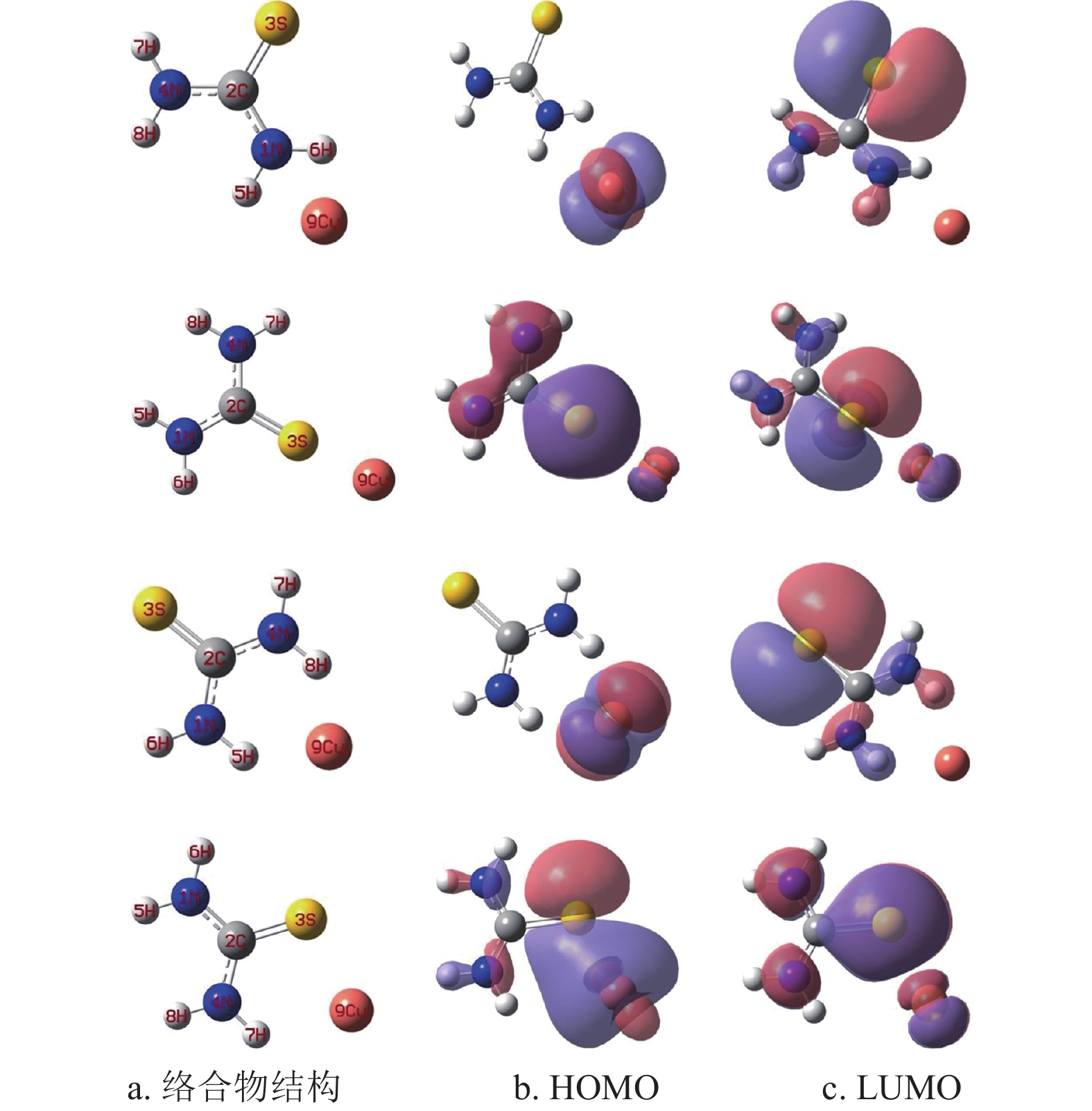
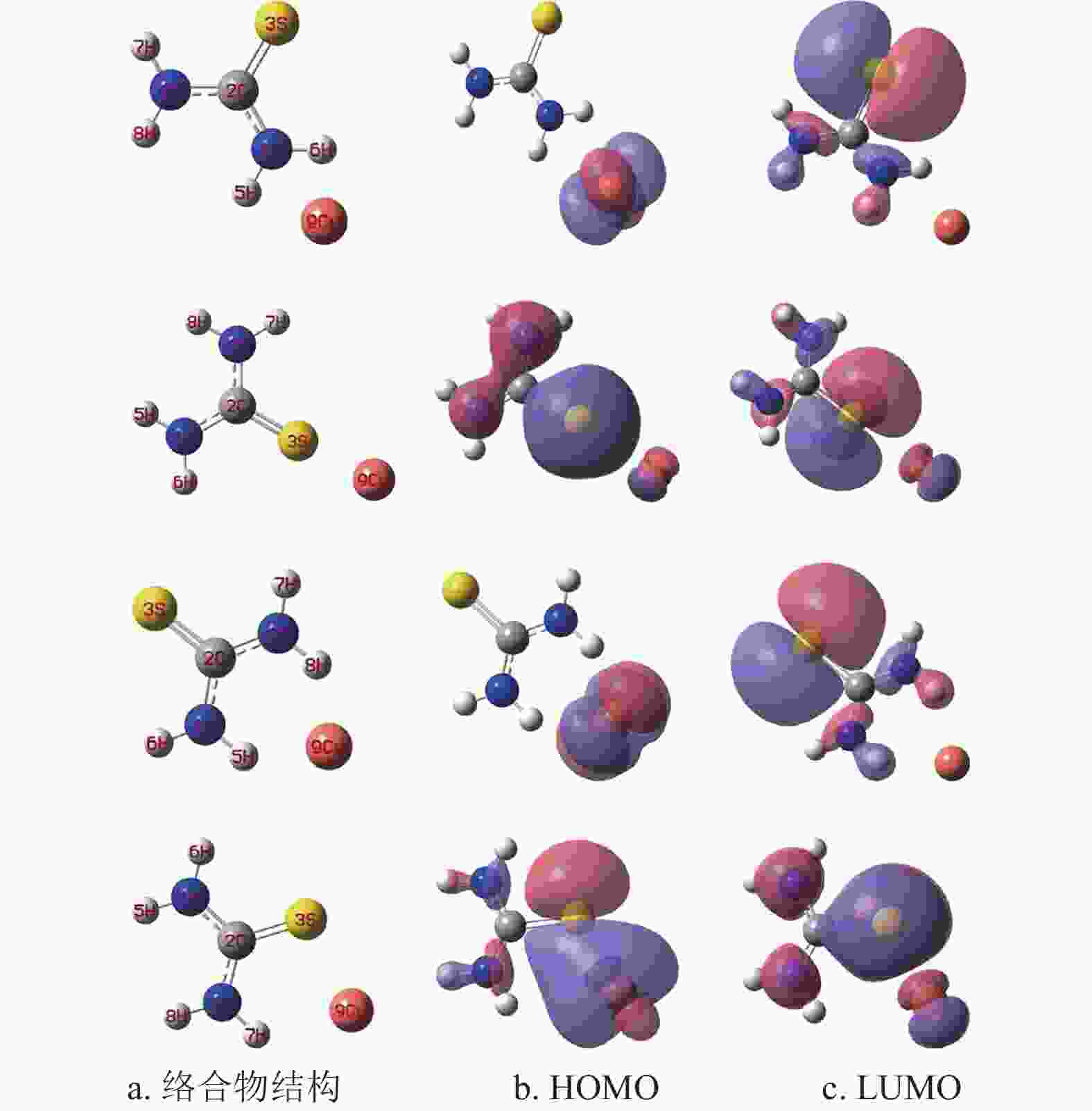
硫脲分子中的N和S原子核外具有孤对电子[19],兼容性Cu2+溶液中的Cu2+理论上优先被吸附在硫脲分子中的N或S原子位置。借助量子化学计算模拟硫脲分子与Cu2+的吸附位点,图2为硫脲分子与Cu2+可能形成的4种络合物结构、最高占据分子轨道(high occupied molecular orbital, HOMO)和最低未占据分子轨道(lowest unoccupied molecular, LUMO)。根据前线分子轨道理论[20-21],分子的HOMO容易给予电子与阳离子形成络合物;分子的LUMO优先从电子给予体获得电子发生化学反应。根据图2可知,4种络合物的HOMO都可以继续给予电子与Cu2+形成新的络合物;同时4种络合物的LUMO也可能继续从硫脲分子获得电子形成新的络合物。络合物中硫脲分子与Cu2+之间的键能通过式(1)进行计算。4种络合物中硫脲分子与Cu2+之间的键能分别为65.726 kJ/mol、87.582 kJ/mol、71.459 kJ/mol和93.465 kJ/mol。计算结果表明,图2中最后一排硫脲分子与Cu2+之间的键能最大,可能是(N─S)协同作用的结果,Cu2+优先被吸附在N和S原子之间。
-
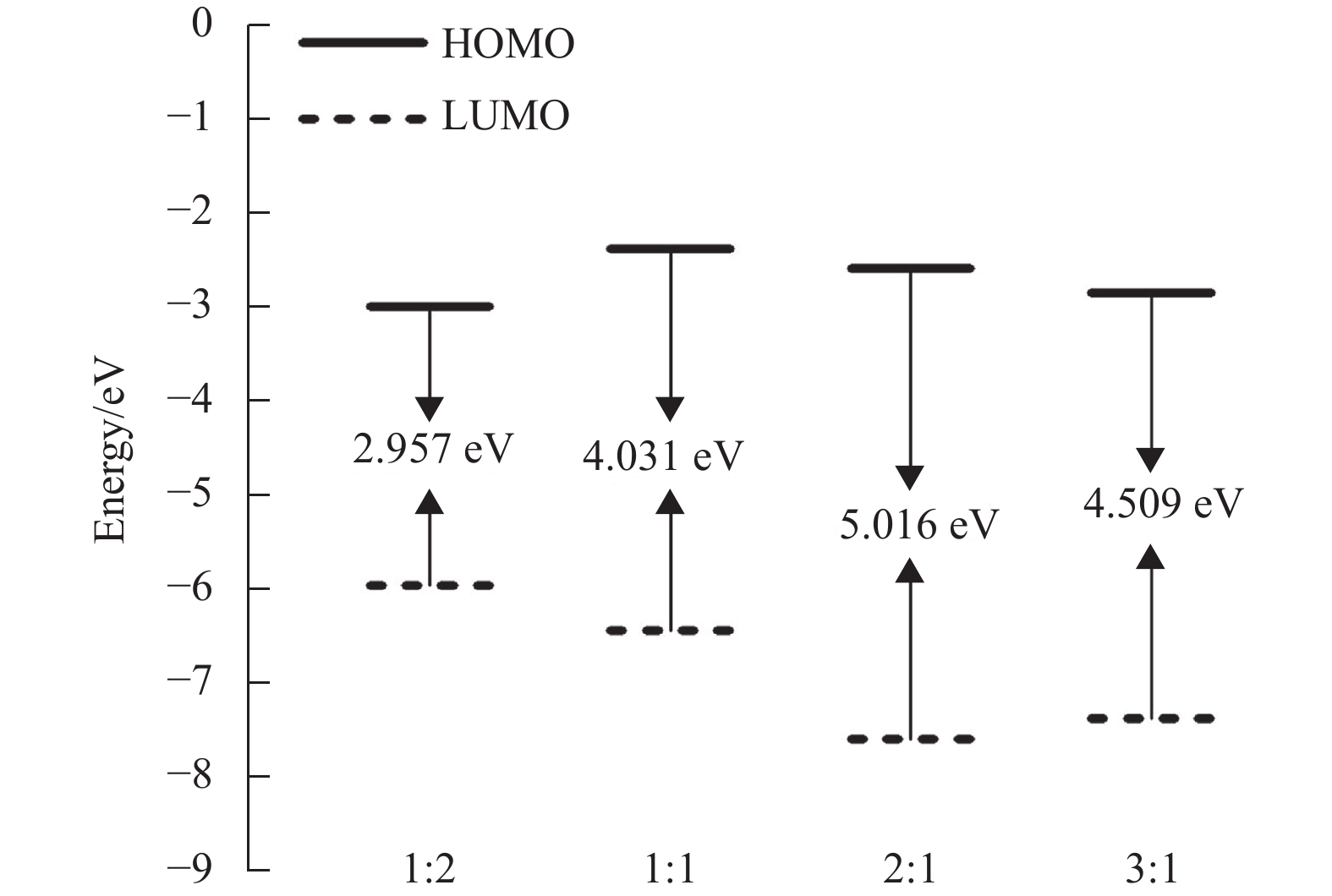
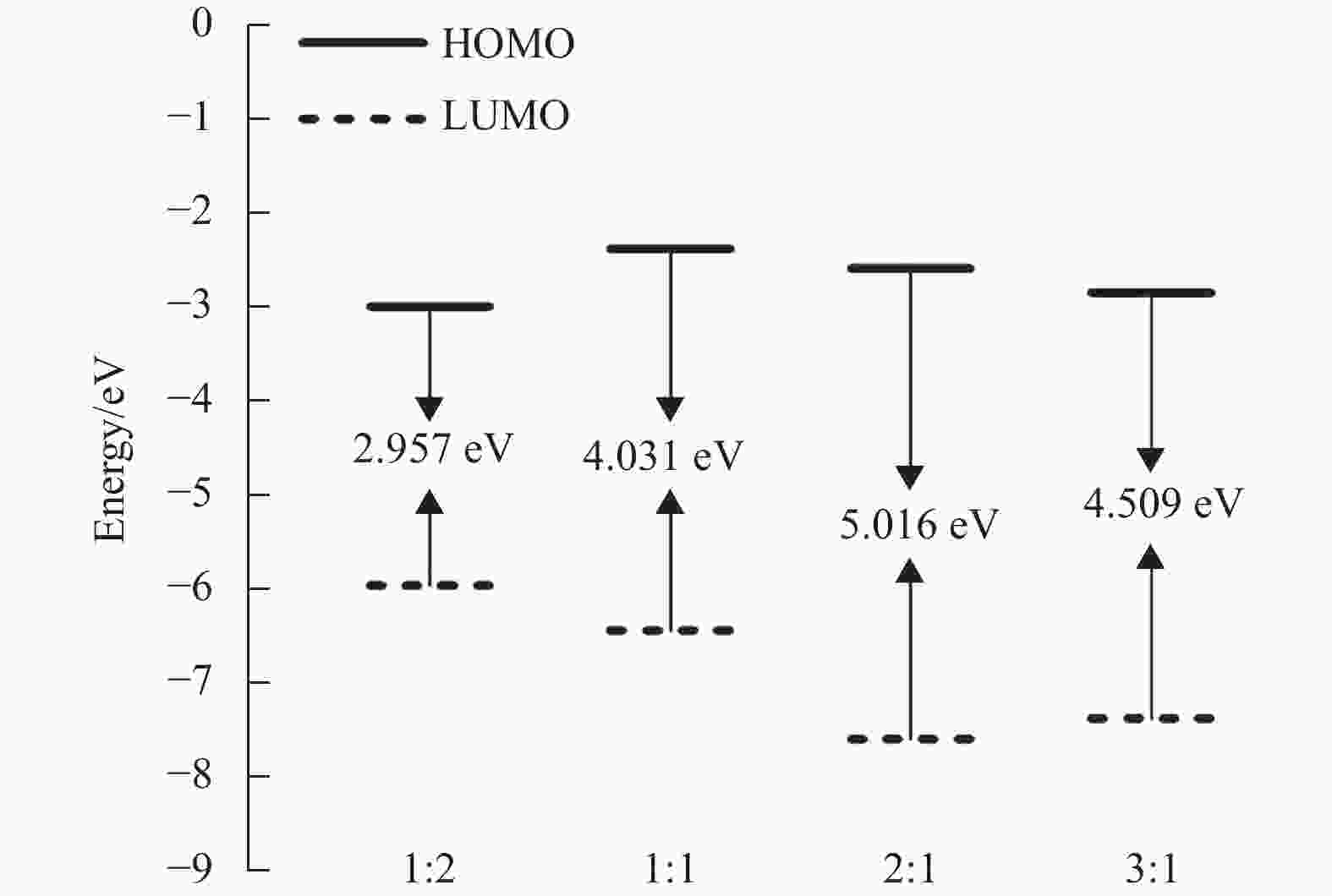
根据量子化学计算可知,当n(硫脲):n(Cu2+)=1:1时,Cu2+优先被吸附在硫脲分子中N和S原子之间,且络合物可以进一步与硫脲分子或Cu2+形成新的络合物,结构最稳定的络合物才能在兼容性Cu2+溶液中稳定存在。不同比例硫脲分子与Cu2+形成络合物的结构稳定性采用密度泛函理论进行模拟计算,络合物的能隙根据公式∆E=
$ {{E}}_{\text{LUMO}}-{{E}}_{\text{HOMO}} $ 进行计算,其能隙∆E越大表明络合物结构越稳定[22-23]。图3为不同比例硫脲分子与Cu2+形成的4种结构优化的络合物前线分子轨道能量示意图,从图中可以看出,4种络合物能隙∆E分别为2.957 eV(1:2)、4.031 eV(1:1)、5.016 eV(2:1)和4.509 eV(3:1)。因此,n(硫脲):n(Cu2+)=2:1时,形成的络合物具有最稳定的结构,可以在兼容性Cu2+溶液中稳定存在。 -
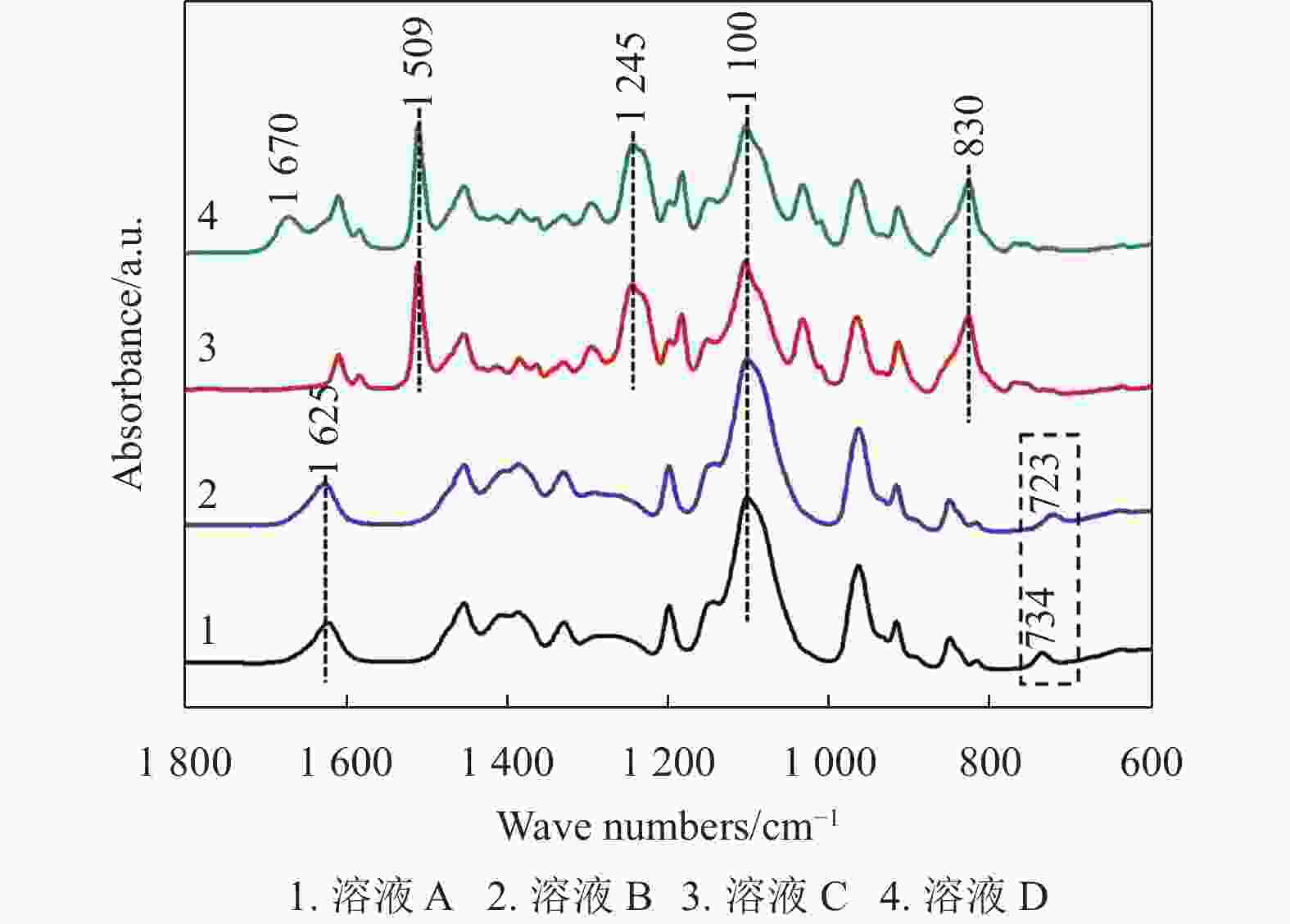
为了研究兼容性Cu2+溶液中各种试剂之间是否发生化学反应,表1按照兼容性Cu2+溶液制备流程列出了不同试剂在丙二醇甲醚中的含量,选用红外光谱法表征兼容性Cu2+溶液中特殊官能团的变化。图4中4条曲线分别对应表1中溶液A、B、C和D的红外吸收光谱图。其中,曲线1中1100 cm−1处的峰值对应丙二醇甲醚中醚键(C─O─C)的伸缩振动[24-25],734 cm−1、1625 cm−1处的两个峰值分别对应(C═S)的伸缩振动和氨基(─NH2)的弯曲振动[26],两个特征官能团(C═S)和氨基(─NH2)说明硫脲溶解于丙二醇甲醚中能够稳定存在。曲线2与曲线1相比几乎没有变化,仅有734 cm−1处的峰值移动到723 cm−1处,这种现象可能是(C═S)基团自发吸附Cu2+后,导致其能量降低而产生红移。曲线3和曲线4是一组对比实验,830 cm−1、1245 cm−1、1509 cm−1处的3个峰值分别对应BADGE中环氧基团(─CH (O)CH─)的伸缩振动、醚键(C─O─C)的伸缩振动和苯环中(C─C)的伸缩振动[27];1670 cm−1处的峰值对应亚胺基团(─NH─)的伸缩振动仅出现在曲线4中,同时1625 cm−1处的峰值对应氨基(─NH2)的弯曲振动在曲线4中消失[28]。因此,根据文献[29]报道推断,硫脲分子中的氨基(─NH2)和BADGE中环氧基团(─CH(O)CH─)发生化学反应,氨基(─NH2)转化为亚胺基团(─NH─)。
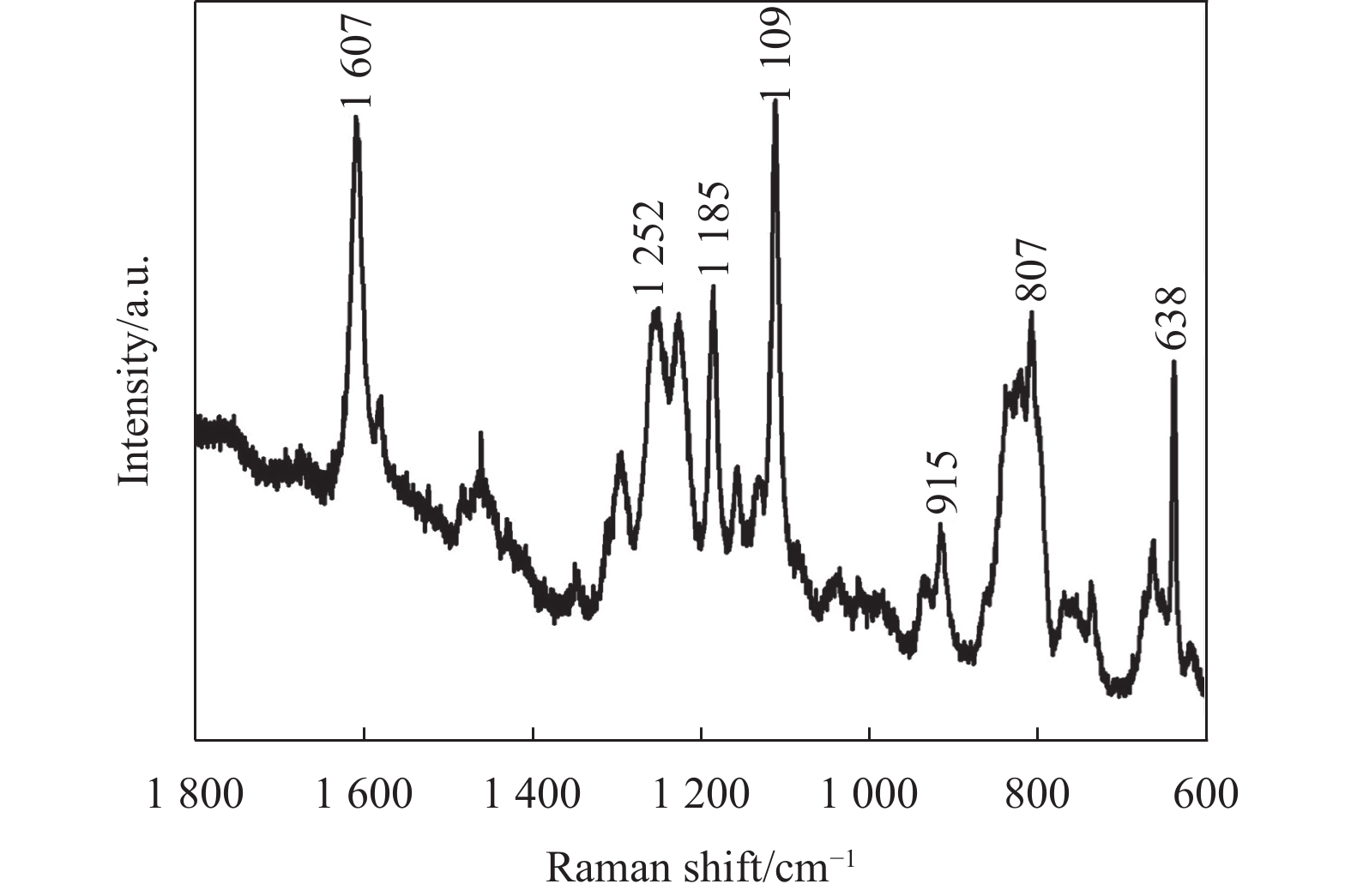
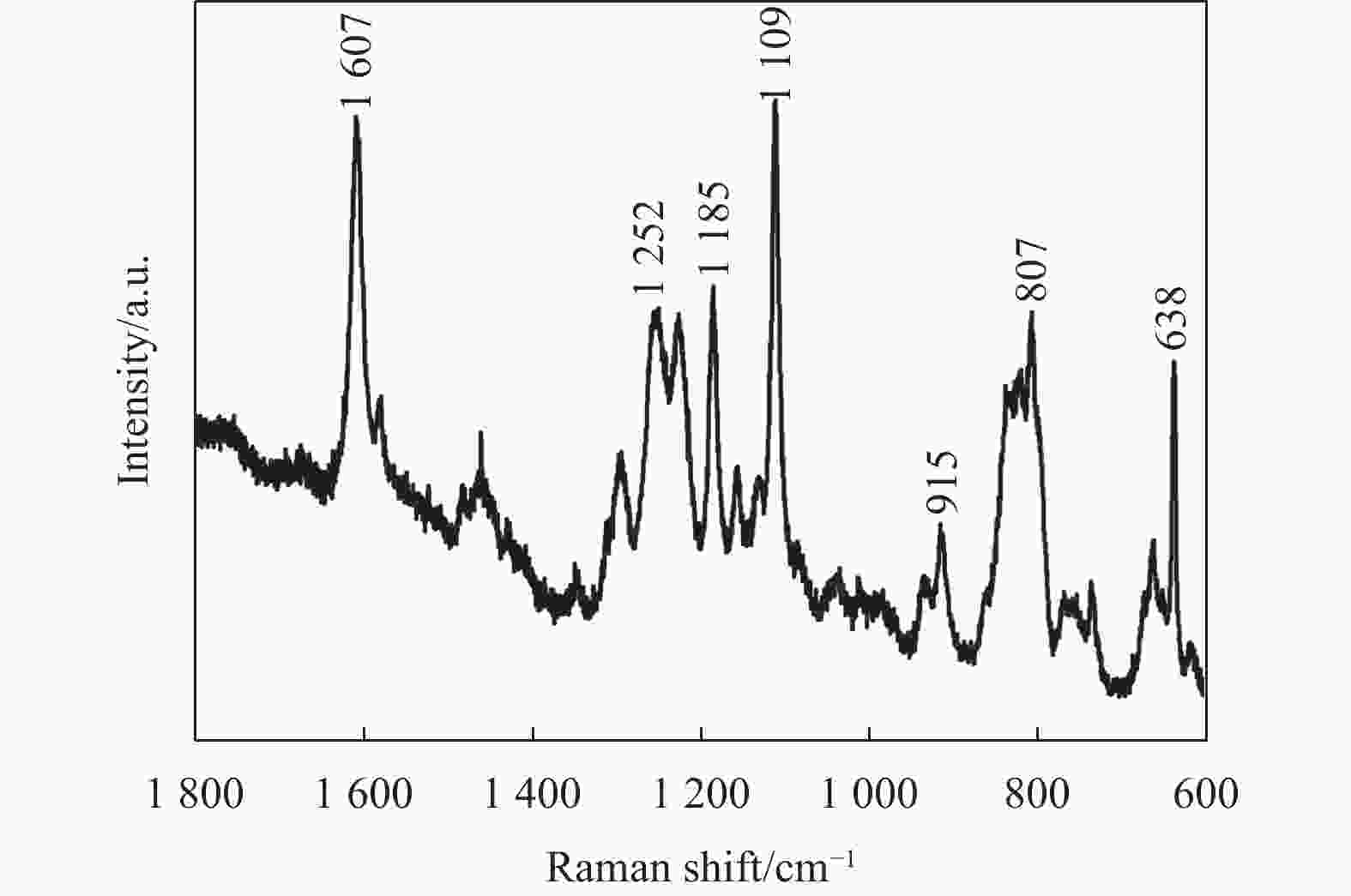
溶液标号 硫脲 乙酸铜 BADGE A 0.4 0 0 B 0.4 0.2 0 C 0 0 2 D 0.4 0.2 2 曲线4中,723 cm−1处的峰值对应(C═S)伸缩振动峰消失,可能是(C═S)在溶液D中与BADGE发生化学反应。拉曼光谱法被用来进一步研究溶液D中官能团的变化。图5为溶液D挥发掉溶剂丙二醇甲醚得到蓝色沉淀的拉曼光谱图,1607 cm−1处对应苯环中(C═C)的伸缩振动峰,1252 cm−1处对应环氧基团(─CH(O)CH─)弯曲振动峰,1185 cm−1处对应苯环中(C─H)的伸缩振动峰,1109 cm−1处对应醚键(C─O─C)伸缩振动峰,915 cm−1处对应环氧基团中(C─O)的伸缩振动峰,807 cm−1处对应(CH2)伸缩振动峰,这6个代表性的振动峰表明蓝色沉淀中含有BADGE[30-32]。另外,1102 cm−1处没有检测到(C═S)伸缩振动峰,而638 cm−1处出现一个新的拉曼光谱峰(C─S─C,伸缩振动)[30, 33]。结合文献[34]报道推论,硫脲分子中的(C═S)与BADGE中的环氧基团(─CH(O)CH─)发生反应生成C─S─C。
根据上述红外光谱和拉曼光谱的分析结果可知,硫脲分子中的氨基(─NH2)、(C═S)能够与BADGE中的环氧基团(─CH(O)CH─)发生反应,分别生成亚胺基团(─NH─)和C─S─C。因此,硫脲分子通过共价键与BADGE结合,同时N和S原子核外的孤对电子能够络合吸附Cu2+,使Cu2+被固定在BADGE上。
-
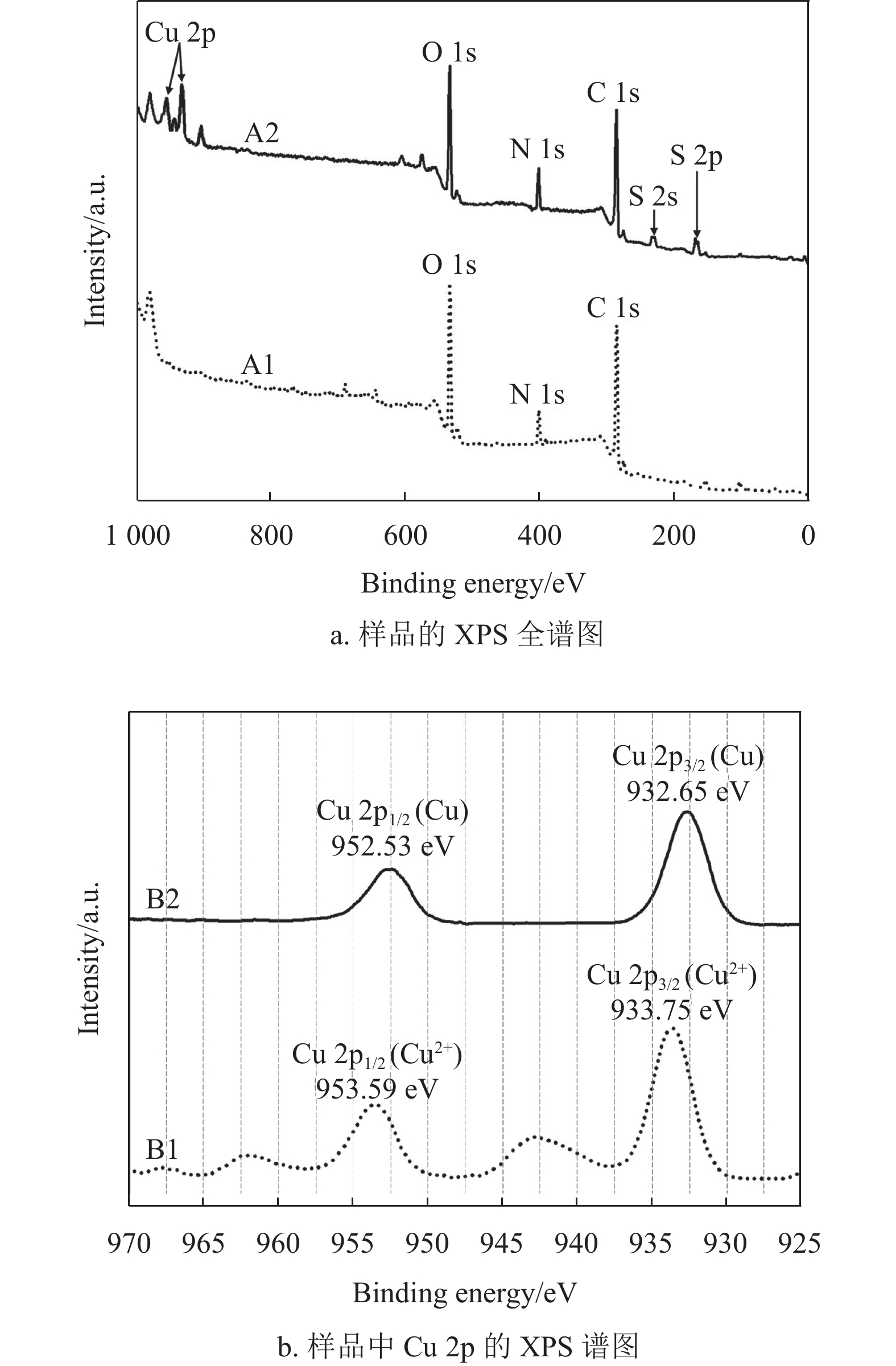
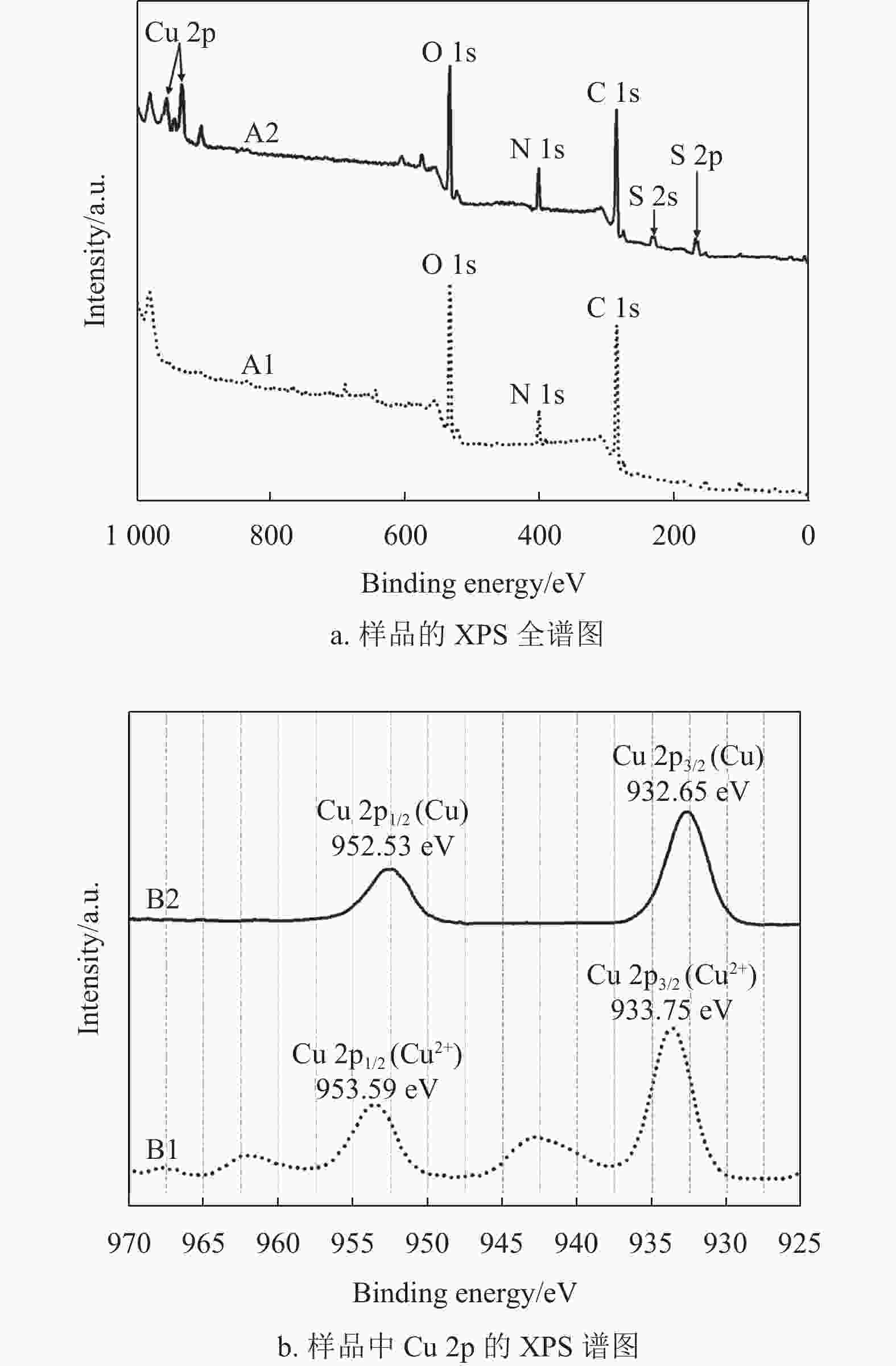
图6a为样品A1(EP基材)和A2(兼容性Cu2+溶液固化后的EP基材)的XPS全谱图,从样品A1的XPS谱图中可以看出,EP基材元素主要包括O 1s (533.85 eV)、N 1s (402.55 eV)和C 1s (286.85 eV)[35],说明实验选用的是固化剂为胺类的常用EP基材。样品A2放入超声水浴中处理10 min后做XPS测试,仍在样品A2中检测到Cu 2p (933.75 eV和953.59 eV)、O 1s (533.85 eV)、N 1s (402.55 eV)、C 1s (286.85 eV)、S 2s (229.65 eV)和S 2p (165.95 eV)[11, 36]。根据图6a的XPS全谱图对比分析表明,兼容性Cu2+溶液固化后形成的改性层能够与EP基材牢固结合,其作为桥接层有利于提高EP基材与铜导电线路之间的结合力。
图6b为样品A2中Cu 2p的XPS谱图,用来表征改性层中铜元素的化合价态。曲线B1是等离子处理前的XPS谱图,两个峰的位置分别在953.59 eV和933.75 eV附近[36]。曲线B2是空气等离子体处理15 min后的XPS谱图,两个峰的位置分别在952.53 eV和932.65 eV附近[37-38]。根据图6b的结果可知,空气等离子体处理15 min后可以把改性层中的Cu2+还原为金属Cu,将其转化为具有启动化学镀铜的活化层。
-
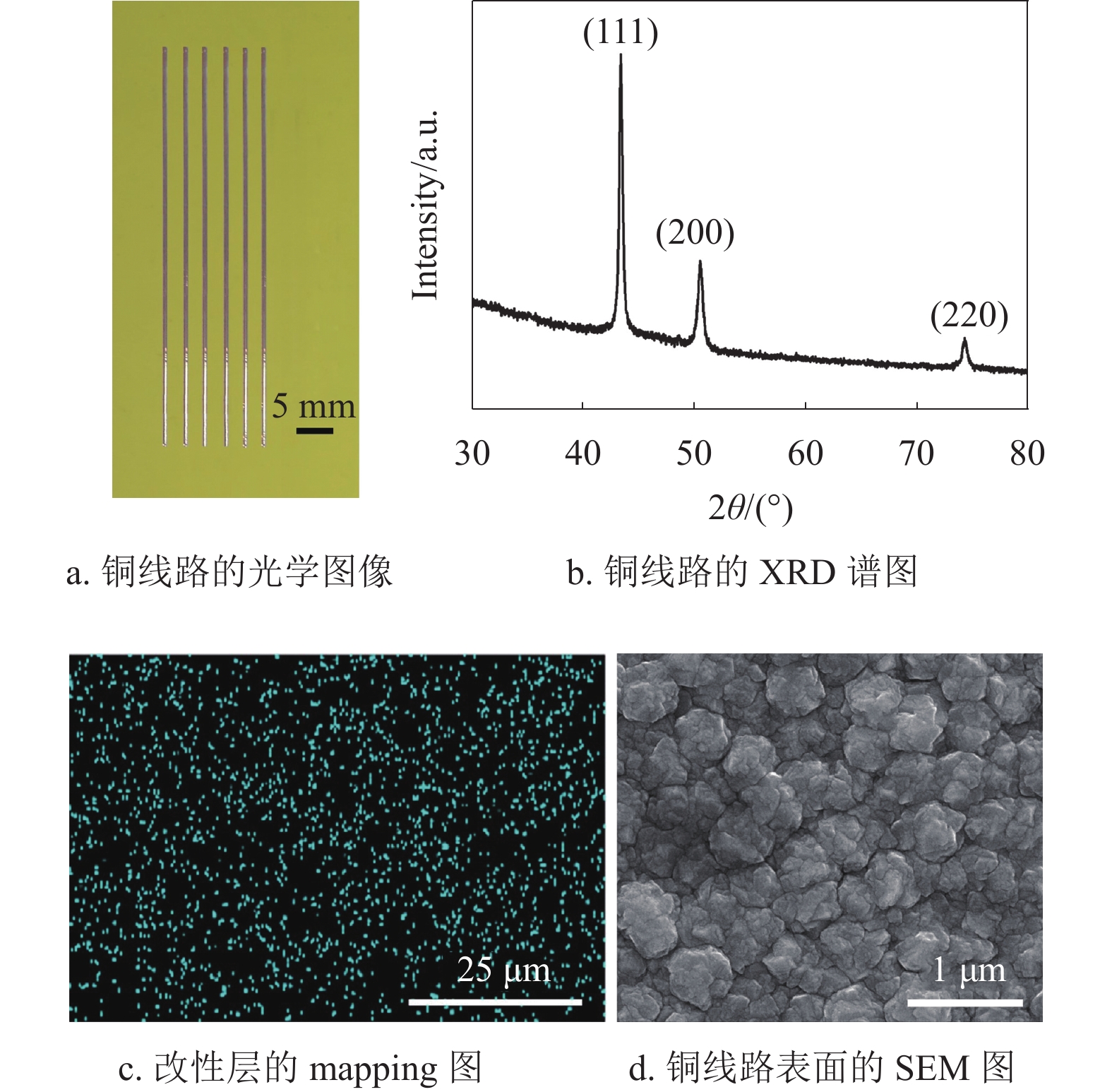
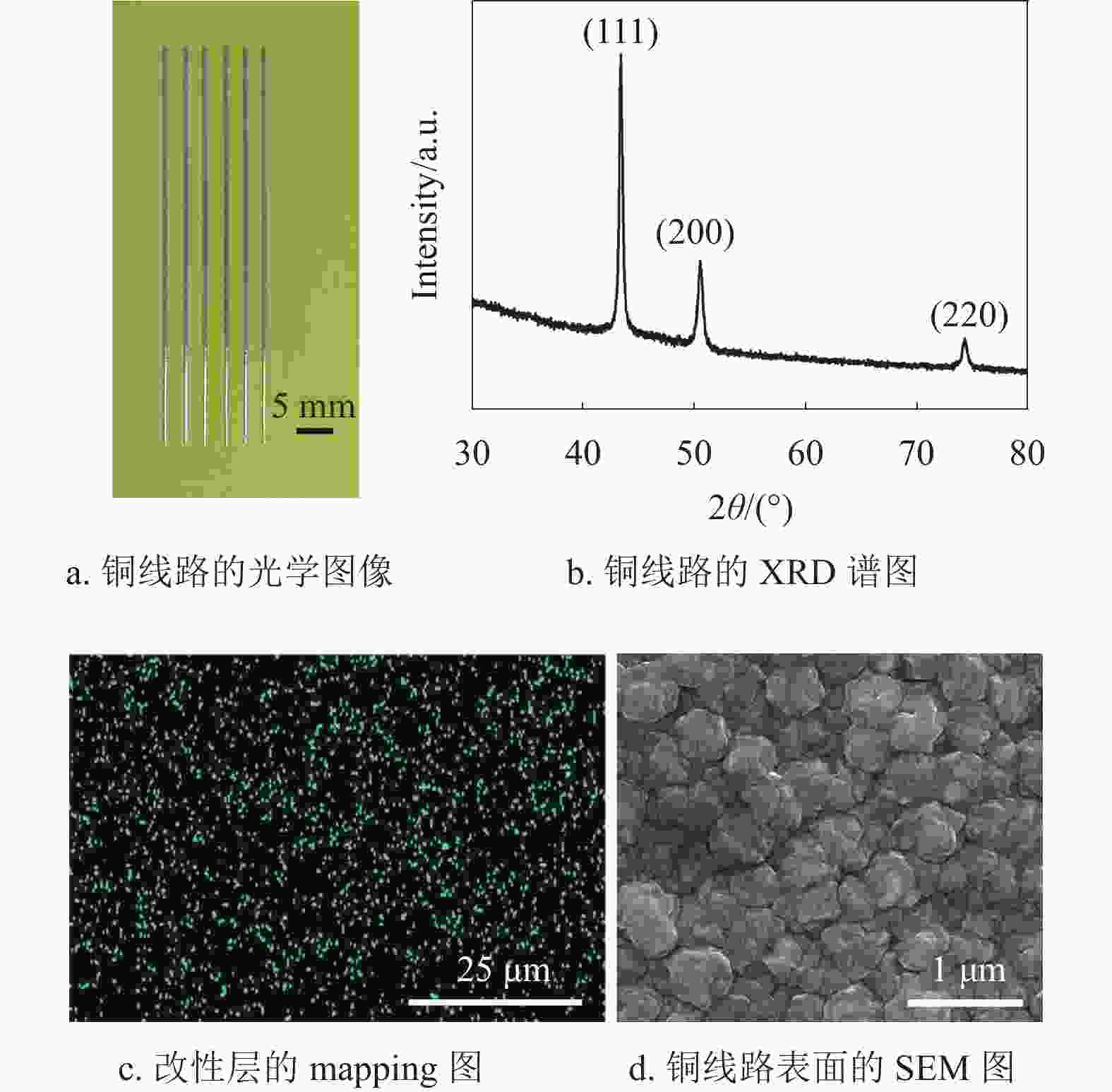
PCB中铜线路用于电子元器件的互连,其导电性能是重要的参考指标之一。图7a为沉积40 min铜线路的光学图像,可以看出铜线路线宽约为550 μm,线路边缘光滑且没有出现渗镀,说明该方法能够在EP基材表面制作线宽均匀的铜线路。图7b为沉积40 min铜线路的XRD谱图,3个布拉格衍射峰的位置在2θ角4.34°、50.7°和74.2°附近,其分别对应面心立方结构Cu的(111)、(200)和(220)晶面[39]。另外,3个尖锐的特征衍射峰表明,铜晶粒结晶度良好,能够有效减小铜的本征电阻,提高铜线路的电导率。图7c为改性层(空气等离子体处理15 min)表面的mapping测试图,可以看出作为催化活性中心的铜纳米颗粒(蓝点)均匀分布在改性层表面,这有利于铜晶粒均匀沉积。图7d为沉积40 min后铜线路表面的SEM形貌图,可以看出沉积的铜晶粒结晶均匀,尺寸约为500 nm,晶粒之间结晶致密且没有孔隙,有利于减小铜线路中晶粒间的接触电阻。
表2为沉积40 min铜线路不同参数的测量值,其中,L、W、D和R分别为铜线路的长、宽、厚度和电阻值。根据电阻率公式
$ \rho ={R}\dfrac{{WD}}{{L}} $ ,计算出铜线路电阻率为2.62×10−6 Ω·cm,其电导率可以达到块状铜的64.1%。参数 L/mm W/mm D/μm R/Ω 测试值 50.03 0.55 2.35 1.0142 -
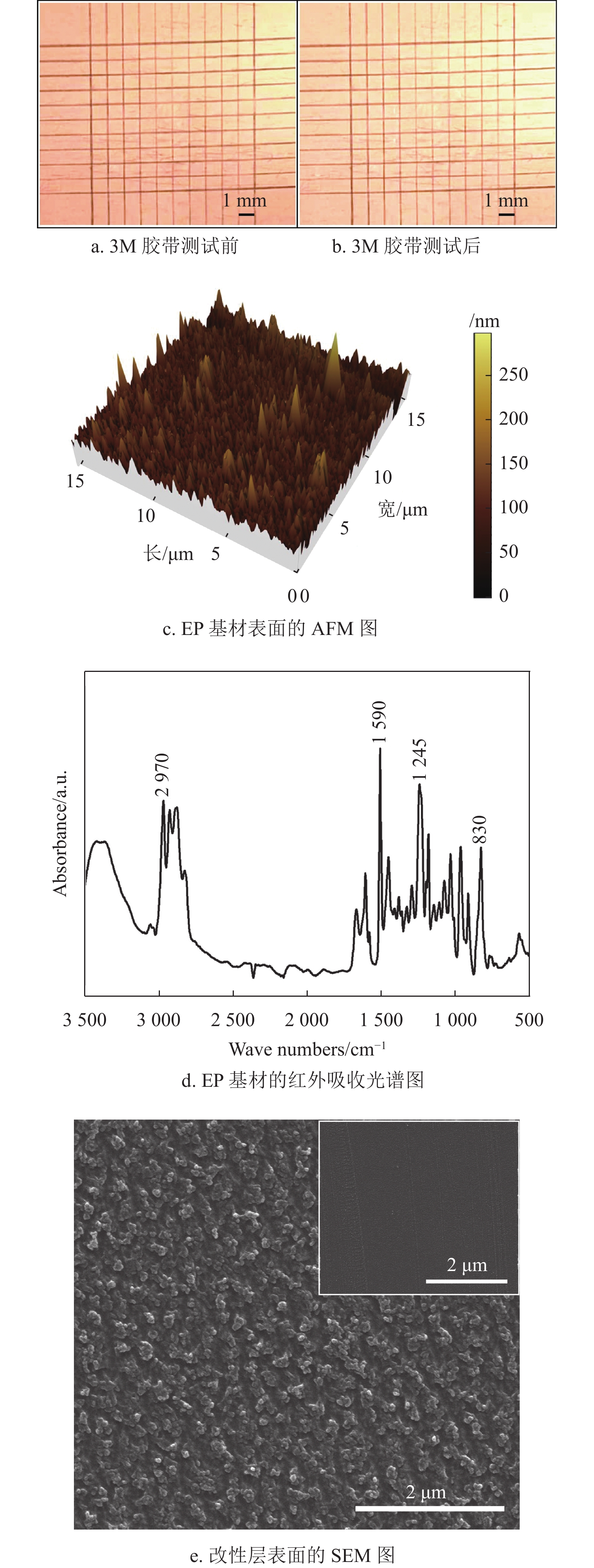
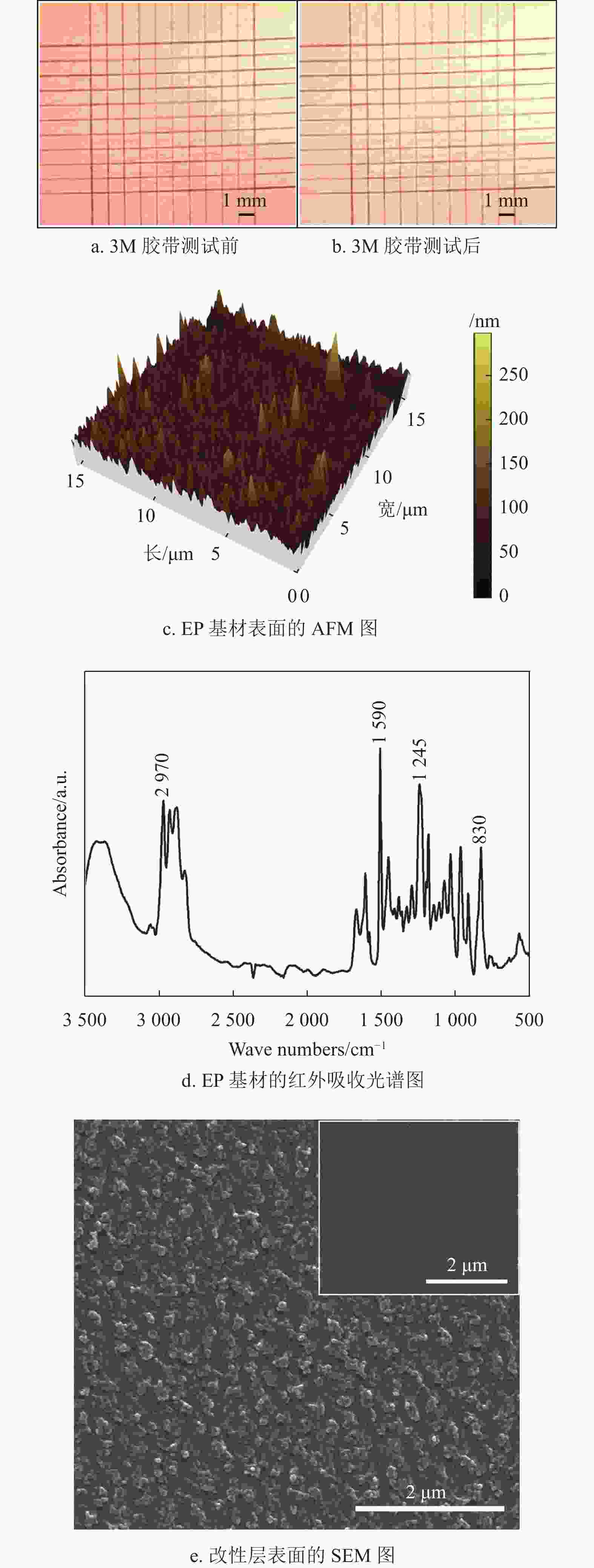
铜导电线路与EP基材之间的结合力是决定PCB使用寿命的一个重要指标。百格测试法用来表征铜线路与EP基材之间的结合力,图8a为3M胶带测试前的光学图像,可以看出百格刀切割后的铜层边缘光滑且平整;图8b为3M胶带测试后的光学图像,可以看出没有铜层从EP基材表面脱落。百格测试结果证明,根据ASTM D3359标准,铜线路与EP基材之间的结合力达到5B等级,表明采用该方法制备的铜导电线路与EP基材之间具有强的结合力。
图8c为用AFM测试EP基材表面的粗糙度,结果表明EP基材的面粗糙度(Sa)约为75 nm,说明EP基材表面非常光滑,其很难与改性层之间形成机械锁扣。然而,百格测试结果表明,铜导电线路与EP基材之间具有强结合力,说明改性层与EP基材之间的结合并不是一种简单的物理结合。图8d为EP基材的红外吸收光谱图,830 cm−1、1245 cm−1、1509 cm−1和2970 cm−1处的4个峰值分别对应环氧基团(─CH(O)CH─)的伸缩振动、醚键(C─O─C)的伸缩振动、苯环中(C─C)的伸缩振动、羟基(─OH)的伸缩振动[11]。红外测试结果表明,EP基材表面有大量的环氧基团(─CH(O)CH─)和羟基(─OH)。值得注意的是,EP基材表面的环氧基团能够与兼容性Cu2+溶液中环氧基团和固化剂593中的氨基(─NH2)发生反应式(2)[29];同时EP基材表面的羟基能够与兼容性Cu2+溶液中的环氧基团发生反应式(3)[40]。因此,改性层与EP基材之间通过化学反应式(2)和式(3)生成的共价键牢固地结合在一起。

图8e中插图是改性层(空气等离子体处理前)表面的SEM图,能够看出改性层表面比较光滑,图8e为经空气等离子处理15 min后改性层表面的SEM图,可以看出其表面变得凹凸不平,这样沉积的铜层就能够与改性层形成机械锁扣提高结合力[41]。另外,结合图6b的XPS分析结果可知,空气等离子体处理后能够把改性层中的Cu2+还原为具有催化活性的金属铜,沉积的铜层会与改性层表面的铜纳米颗粒形成金属键。因此,改性层与铜层通过机械锁扣和金属键牢固地结合在一起。
根据图8b、8c和8d的分析结果可知,改性层作为桥接层通过物理机械锁扣和金属键把EP基材与铜层牢固地结合在一起,分析结果与图8a的百格测试结果一致。
-
本文设计出一种基于EP基材兼容的Cu2+溶液,采用选择性化学镀铜法制备了性能优异的铜导电线路。主要结论如下:1)量子化学计算结果表明,改性溶液中n(硫脲):n(Cu2+)=2:1时,形成的络合物结构最稳定;2) XPS测试结果证明,空气等离子处理15 min后,改性层中的Cu2+被还原为金属Cu;3)导电性能分析结果表明,化镀40 min后铜线路电阻率低至2.62×10−6 Ω·cm;4)结合力分析结果表明,改性层通过物理机械锁扣和金属键把铜线路与EP基材牢固地结合在一起,使结合力达到5B等级。总之,EP基材兼容性改性催化铜导电线路沉积工艺简单、满足绿色生产,制备的铜线路性能优异,对其他常用树脂基材表面兼容性改性加成制备PCB具有一定的参考价值。
本文研究工作得到运城学院博士科研启动项目(YQ-2021009)和运城学院学科建设经费的支持,在此表示感谢。
Modification Epoxy Resin Substrate with Compatible Cu2+ Solution to Catalyze Copper Circuits Deposition
doi: 10.12178/1001-0548.2022065
- Received Date: 2022-03-07
- Accepted Date: 2022-07-01
- Rev Recd Date: 2022-05-17
- Available Online: 2022-11-28
- Publish Date: 2022-11-25
-
Key words:
- compatible Cu2+ solution /
- density functional theory /
- epoxy resin /
- printed circuit board /
- selective electroless copper plating /
- surface modification
Abstract: The predesigned position activation of printed circuit board (PCB) substrates is the key process for manufacturing circuits by selective electroless copper plating. A compatible Cu2+ solution for epoxy resin (EP) substrate was designed with copper acetate monohydrate as a catalyst precursor, thiourea as a complexing agent, bisphenol A diglycidyl ether as a prepolymer of EP, reagent 593 as a curing agent, and 1-methoxy-2-propanol as a solvent. The compatible Cu2+ solution was printed on EP substrate surface by an inkjet printer. Copper circuits were additively fabricated by selective electroless copper plating. Based on quantum chemistry density functional theory, the complexation reactions between thiourea molecules and copper ions were simulated in the compatible Cu2+ solution. The special functional groups in the compatible Cu2+ solution were characterized by infrared spectroscopy and Raman spectroscopy. The results show that the resistivity of copper circuits is as low as 2.62×10−6 Ω·cm attributing to the good crystallization and dense accumulation of copper grains. The adhesion between copper circuit and EP substrate is up to 5B level with the help of the modified layer. Therefore, compatible modification EP substrate to catalyze copper circuits deposition has the advantages of simple process, economic and environmental-friendly, which provides a valuable reference for compatible modification on other common resin substrates in additive manufacturing of PCB.
| Citation: | WANG Yuefeng, HONG Yan, JI Linxian, ZHANG Cun, MA Ziwei. Modification Epoxy Resin Substrate with Compatible Cu2+ Solution to Catalyze Copper Circuits Deposition[J]. Journal of University of Electronic Science and Technology of China, 2022, 51(6): 953-960. doi: 10.12178/1001-0548.2022065 |

 ISSN
ISSN 










 DownLoad:
DownLoad: